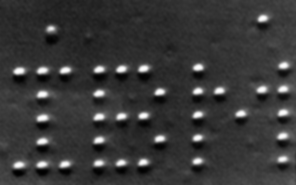
In 1989 a physicist at IBM named Donald Eigler and his colleague Erhard Schweizer used a scanning tunneling electron microscope in a new way. Instead of (merely!) looking at individual atoms, they modified the instrument so that they could move individual atoms. This was the result:
I don’t really know why, but I would guess it’s because xenon is non-reactive and the heaviest of the so-called “noble” gases. The elements on the far right of the periodic table (helium, neon, argon, krypton, xenon, and radon) are mostly inert. They don’t do much reacting with other elements. Radon is radioactive and one of the rarest elements, so even though its atomic mass is 222, it can’t be worked with. Xenon is the next-heaviest (131).
Xenon occurs naturally in the atmosphere in very small amounts. But it’s a big atmosphere so there is actually a lot of the stuff!
These days it is used as a general anesthetic. Related to that, xenon is used in breathing mixtures for deep-sea divers.